Not for my German pils…it’s essential
The world will become a much more pleasant place to live when each and every one of us realizes that we are all idiots.
Yep
Pope of Dope:
Thanks, everybody.
Living in Los Angeles I’ve made some bad tap water batches. There’s only so much you can do with it, and when it comes to the lighter styles, it comes out dirty tasting.
Didn’t know about the chalk, that is good to know, and to be a jerk about it, why Marin is it even listed as a mineral addition if it is ultimately ineffective?
I have no deep wells. So best I can do about my dilemma is to go to Ralphs and buy RO water in gallon jugs. My new water additions look like this:
.9g gypsum
.2g cal chlor.
5 ml Lactic.It gives me a pretty close profile to Boiled Munich.
Gypsum is wicked out of place in German beer.
Sent from my iPhone using Tapatalk
Not for my German pils…it’s essential
Which is I am assuming… single infused, late hopped, fermented with dry yeast, warmer?
Again thats totally fine, but nothing a real German brewery would do.
But in the spirit of RDWHAHB, I shall bow out of this… You guys do you.
denny:
Pope of Dope:
Thanks, everybody.
Living in Los Angeles I’ve made some bad tap water batches. There’s only so much you can do with it, and when it comes to the lighter styles, it comes out dirty tasting.
Didn’t know about the chalk, that is good to know, and to be a jerk about it, why Marin is it even listed as a mineral addition if it is ultimately ineffective?
I have no deep wells. So best I can do about my dilemma is to go to Ralphs and buy RO water in gallon jugs. My new water additions look like this:
.9g gypsum
.2g cal chlor.
5 ml Lactic.It gives me a pretty close profile to Boiled Munich.
Gypsum is wicked out of place in German beer.
Sent from my iPhone using Tapatalk
Not for my German pils…it’s essential
Which is I am assuming… single infused, late hopped, fermented with dry yeast, warmer?
Again thats totally fine, but nothing a real German brewery would do.But in the spirit of RDWHAHB, I shall bow out of this… You guys do you.
Wow. Just reminded of this exchange last year.
https://www.homebrewersassociation.org/forum/index.php?topic=32026.msg414329#msg414329
Guess the heat’s getting to us the same way again, eh fellas?
Nope, no beef.
The proverbial you, do you. I’ll do me. I forgot were I was for a minute.
…You yourself have griped in the past about how the AHA website isn’t always accurate or current, but yet it should be the go-to place for accurate and current homebrewing knowledge. …
I’ve said this ^^^^.
denny:
Pope of Dope:
Thanks, everybody.
Living in Los Angeles I’ve made some bad tap water batches. There’s only so much you can do with it, and when it comes to the lighter styles, it comes out dirty tasting.
Didn’t know about the chalk, that is good to know, and to be a jerk about it, why Marin is it even listed as a mineral addition if it is ultimately ineffective?
I have no deep wells. So best I can do about my dilemma is to go to Ralphs and buy RO water in gallon jugs. My new water additions look like this:
.9g gypsum
.2g cal chlor.
5 ml Lactic.It gives me a pretty close profile to Boiled Munich.
Gypsum is wicked out of place in German beer.
Sent from my iPhone using Tapatalk
Not for my German pils…it’s essential
Which is I am assuming… single infused, late hopped, fermented with dry yeast, warmer?
Again thats totally fine, but nothing a real German brewery would do.But in the spirit of RDWHAHB, I shall bow out of this… You guys do you.
You know what they say about assume…you are incorrect on all counts. And what I care about anyway is results, not process. If it tastes like a good German pils, I don’t care how I get there.
Wow!! I’m Marin now?? I’m hoping my name fits me to a T.
Chalk certainly is usable in brewing and its not a mistake to include that option in a calculator. The real problem is that users don’t understand how difficult it is to dissolve chalk in water. Unless the user is employing pressurized CO2 to supply the carbonic acid necessary to achieve solubility, its not going to work as the brewer assumes or intends. It appears that I should incorporate some sort of pop up warning when a user puts a value in the chalk addition cell.
The other thing that needs to be pointed out is that many Bru’n Water profiles include the alkalinity (presented as bicarbonate in Bru’n Water) that would be present in the raw water from their water source. In many cases, the brewers of old had to implement techniques or additions to neutralize that alkalinity. That is the case in Bru’n Water too. While you could ADD that alkalinity to create a more authentic starting water profile, you would almost certainly have to neutralize it in the mashing and sparging water to produce a desirable beer. I suggest that brewers ignore the bicarbonate and calcium targets that are presented in water profiles and only add the minerals and acids necessary to produce an acceptable mashing pH and low sparging water alkalinity. The rest of the water profile ion concentrations are what you should consider your real targets.
Sulfate is indeed a very useful and DESIRABLE component in brewing German beers. One only has to taste a good German Pils or Kolsch to know that sulfate is an important feature in their overall beer perception. Even in southern Bavarian styles, the low sulfate content provides a slight drying in the beer’s finish to help them avoid being cloying or overly full. Unfortunately, there are under-educated individuals that continue myths such as sulfate causes undesirable effects with noble hops or sulfate will create bitter beer. I can only state that they are incorrect in their perceptions and admissions. There is room for PROPER sulfate levels in German beer brewing. It should be low in Bavarian styles.
Thanks Marin. ![]() The more you know.
The more you know.
To hijack my own post, got busy and messed up the volume and ended up with 1.042 OG (which probably gives me a 3.5 abv). Anyone else brew a Hef like this before? My concern is that it’s going to be watery.
To hijack my own post, got busy and messed up the volume and ended up with 1.042 OG (which probably gives me a 3.5 abv). Anyone else brew a Hef like this before? My concern is that it’s going to be watery.
Have any DME hangin around? You could bump up the OG.
I’ve done it using Kai’s method — once.
http://braukaiser.com/wiki/index.php/Building_brewing_water_with_dissolved_chalk
Interestingly, though Mr deLange is cited as a reference in Kai’s article, he does not recommend it now. He started talking way over my head very quickly but the gist was: don’t use chalk.
Sent from my iPad using Tapatalk
A.J. has the tendency to inadvertently go way over peoples heads!
He’s a fantastic resource though once you understand what he’s talking about.
^^^^
Palmer and Kaminski address, slightly nearer the level of most of our heads, how chalk dissolved this way is very unstable in solution. It is quick to precipitate back out, on its own or by apatite reactions in the mash, and is also very slow to adjust pH (taking several hours to have anything close to the expected effect, which is reversible.)
I think all of us lay people can get an idea of how hard it is to get carbonate into water and how easy to get it out, even when it was dissolved under the uneproducible natural conditions of very high CO2 pressure over geologic time: We all know that just bringing water to the boil does the trick. But even more illustrative is that a simple drop in pressure, as from line pressure to atmospheric pressure, will cause it to break out of solution. That’s how you get lime scale on a faucet. Surely I’d expect opening your 2 liter of Kai-style carbonated water would initate the process.
A.J. of course is one of their sources.
“Don’t use chalk” is a very sensible rule.
Pope of Dope:
…
.9g gypsum
.2g cal chlor.
5 ml Lactic.…
Gypsum is wicked out of place in German beer.
Sent from my iPhone using Tapatalk
Not for my German pils…it’s essential
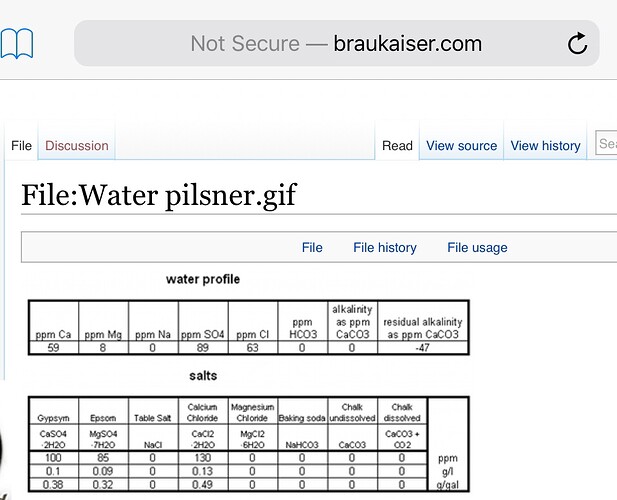
Many probably follow this advice: Sulfate and noble hops don’t tend to play well together, so calcium chloride (not much) to distilled water for a great pilsner. Many would say no gypsum for this reason. However, it looks like Kai is on the gypsum team for a Pils as well:
denny:
Pope of Dope:
…
.9g gypsum
.2g cal chlor.
5 ml Lactic.…
Gypsum is wicked out of place in German beer.
Sent from my iPhone using Tapatalk
Not for my German pils…it’s essential
Many probably follow this advice: Sulfate and noble hops don’t tend to play well together, so calcium chloride (not much) to distilled water for a great pilsner. Many would say no gypsum for this reason. However, it looks like Kai is on the gypsum team for a Pils as well:
Sent from my iPad using Tapatalk
We all have our preferences.
denny:
Pope of Dope:
…
.9g gypsum
.2g cal chlor.
5 ml Lactic.…
Gypsum is wicked out of place in German beer.
Sent from my iPhone using Tapatalk
Not for my German pils…it’s essential
Many probably follow this advice: Sulfate and noble hops don’t tend to play well together, so calcium chloride (not much) to distilled water for a great pilsner. Many would say no gypsum for this reason. However, it looks like Kai is on the gypsum team for a Pils as well:
Sent from my iPad using Tapatalk
Interesting. Kai’s finished profile is very close to mine, as it stands at the moment, for pale lagers. I find sulfate desirable in just about any beer, as long as it’s somewhere under 100ppm. I’ve found I’m less tolerant of chloride. (And I’m not as scared of sodium as I was before letting experience replace dogma, I have a little more than Kai.) So gypsum will probably always have a place even with SMB contributing some potential sulfate, as I’m aiming for ~60ppm calcium. Palates will differ.
Dwain:
denny:
Pope of Dope:
…
.9g gypsum
.2g cal chlor.
5 ml Lactic.…
Gypsum is wicked out of place in German beer.
Sent from my iPhone using Tapatalk
Not for my German pils…it’s essential
Many probably follow this advice: Sulfate and noble hops don’t tend to play well together, so calcium chloride (not much) to distilled water for a great pilsner. Many would say no gypsum for this reason. However, it looks like Kai is on the gypsum team for a Pils as well:
Sent from my iPad using Tapatalk
We all have our preferences.
I prefer a dry crisp finish in a Pils, ala Jever, Koenig, Herren Pils (decreasing dryness).
There are some bland German Pilsners.
I’ve yet to run any good water experiments and I know the vast majority of us are still in the same boat. I mean, you’d have to do blind triangles to know for sure if it even matters. We fool ourselves into thinking things are very important when in reality… well who knows.
Anyone care to comment on this yet? How many of ya’ll have run blind triangles with different water to really know what it does?
Dave Taylor:
I’ve yet to run any good water experiments and I know the vast majority of us are still in the same boat. I mean, you’d have to do blind triangles to know for sure if it even matters. We fool ourselves into thinking things are very important when in reality… well who knows.
Anyone care to comment on this yet? How many of ya’ll have run blind triangles with different water to really know what it does?
No blind triangles, not gonna do it. I’ve learned over time I don’t like anything in excess, but everything in moderation. As long as no single ion approaches 100 ppm I probably will find the water’s contribution balanced and unobtrusive. That’s either useless guidance, or the most helpful possible. It serves me as a guideline so I can concentrate on getting adequate calcium to satisfy the needs of the mash, which is IMHO all that really matters.
Dave Taylor:
Dave Taylor:
I’ve yet to run any good water experiments and I know the vast majority of us are still in the same boat. I mean, you’d have to do blind triangles to know for sure if it even matters. We fool ourselves into thinking things are very important when in reality… well who knows.
Anyone care to comment on this yet? How many of ya’ll have run blind triangles with different water to really know what it does?
No blind triangles, not gonna do it. I’ve learned over time I don’t like anything in excess, but everything in moderation. As long as no single ion approaches 100 ppm I probably will find the water’s contribution balanced and unobtrusive. That’s either useless guidance, or the most helpful possible. It serves me as a guideline so I can concentrate on getting adequate calcium to satisfy the needs of the mash, which is IMHO all that really matters.
That works for me! Cheers!